
Bomb Couriers
Our G4-containing aptamers provide extra plasma stability in terms of biomedical application. Our results also indicated incorporation of chemotherapy drug to the nucleotide backbone of aptamers is an effective approach to lower treatment effective dose (> 10 folds), hence reducing side effects.

Selection of EBV-targeted aptamers
Aptamer-2019 & Aptamer-1194
Our company used a modified SELEX process called Cell-SELEX for selecting our aptamers. Using EBV- positive cells or viral oncoprotein latent membrane protein 1 (LMP1)-positive cells as the target for aptamers selection, we were able to identify aptamers that specifically bind to our EBV-related targets on cell surface in its native confirmation. With combination to in vivo-SELEX, the identified aptamers can be further selected based on their affinity and their accumulative potential to EBV-positive tumors in mice model.

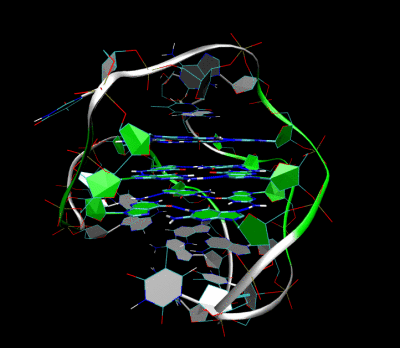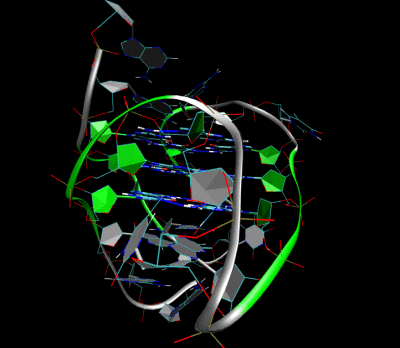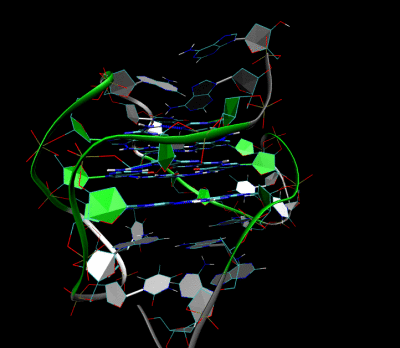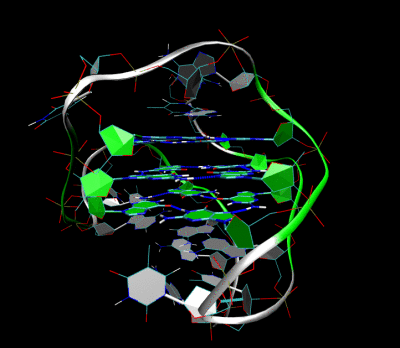
Figures illustrating the formation of G-quadruplex in aptamers sequence.

Our aptamers are G4- forming sequences with high resistance to nuclease degradation, giving high stability to reach the cancer sites.
Our aptamers have high serum stability
idk yet
Aptamer-2019 (Apt-2019) and Aptamer-1194 (Apt-1194) were discovered for targeting to EBV- positive cells. Our characterization studies revealed that Apt-2019 and Apt-1194 are both guanine-rich oligonucleotides (GROs) in which their G content are 43 and 41% respectively. Guanine bases in GROs can form square planner structures called G-quartets through Hoogsteen base pairing, and these G-quartets spontaneously assemble into four-stranded helical structures termed G-quadruplex (G4). G4-forming oligonucleotides are relatively stable against nuclease degradation, thus having high serum stability to Apt-2019 and Apt-1194 for reaching tumor sites.


Apt-2019 and Apt-1194 could be specifically targeted to Xenograft-C15 and C666-1 tumors respectively
Our aptamers can accumulate at the tumor sites
Section Subtitle
Importantly, we have labelled the aptamers with fluorescence to enable imaging of their biodistribution in mice models. Fluorescence-tagged Apt-2019 and Apt-1194 were tail vein injected in mice bearing C15 tumors (a NPC xenograft with high expression of LMP1) and EBV-positive C666-1 tumors respectively. We have also generated control aptamers to Apt-2019 and Apt-1194 by replacing all the G to C, so as to destroy the G4-forming ability. The resulting control aptamers are named as Apt-NG2019 and Apt-NG1194. Tumors and organs (e.g. lungs and spleen) were taken out for fluorescence imaging (IVIS Spectrum imager). Representative images demonstrating the accumulation of Apt-2019 or Apt-1194 (but not the control Apt-NG aptamers) in the tumors were shown in the figure.



Aptamers become couriers of chemodrugs when their cytosine or thymidine nucleotides are replaced to gemcitabine or 5-FU respectively.
Chemodrugs can be incorporated into our aptamers
Section Subtitle
Chemotherapy drugs, such as gemcitabine (Gem) and fluorouracil (5-FU), that have a similar structure to natural nucleotide can be enzymatically incorporated inside the aptamer DNA strand. These therapeutic drugs can be released in situ after hydrolysis of the aptamers and escape from the lysosome by active nucleotide transporters, such as equilibrative nucleoside transporter 3 (ENT3). The conjugation of Gem and 5-FU into our EBV-targeting aptamers creates bifunctional aptamers with both enhanced binding affinity and toxicity. With the replacement of all the T nucleotides to 5-FU in the apt-2019 and C nucleotides to Gem in Apt-1194, our data suggested that drug-loaded Apt-2019 and Apt-1194 were cytotoxic to the NPC cells with an IC50 around 50 nM.

Loading the 5FU into the Apt-2019 could greatly downregulate the effective treatment dose by >10 folds. Mice bearing NPC xenograft (Xeno113) were treated with PBS, Free 5FU and Apt-2019- 5FU via tail vein injection.
5-FU loaded aptamers have higher potency compared to free-5FU
Section Subtitle
Apt-2019 contains 13 Thymidine nucleotides, and we have exchanged them all to 5FU. To demonstrate that Apt-2019 could enhance the delivery of 5FU into the targeted tumor, we have used a dose of 5FU which is 11.5 folds less than the normal treatment dose. The same molar concentration of 5FU was used in the free 5FU and Apt-2019-5FU treatment groups. The free 5FU could not significantly suppress the tumor growth, while the Apt-2019-5FU could suppress the tumor growth.

Apt-2019-5FU showed potent inhibitory effect of the growth of XenoC15, which is known to have high expression of LMP1
5-FU loaded aptamers reduce the effective dosge to 1/10
Section Subtitle
We have also repeated the experiment in one more NPC tumor in vivo model. This xenograft (XenoC15) is known to express a high level of EBV-encoded latent membrane protein 1 (LMP1). Indeed, our Apt- 2019-5FU showed a very potent and significant effect in suppressing tumor growth using only 1/10 concentration of the effective dose of free-5FU.
Conclusion
Section Subtitle
In short, using our 5FU-loaded Apt-2019, it can lower the in vivo effective dose of 5FU by 10 folds compared to free 5FU treatment. There is no observable change in the morphology of the spleen, liver and kidney and body weight of mice between the control and treatment groups. This implies the 5FU- loaded Apt-2019 did not impose adverse effects on the mice. All these results showed that Apt-2019 could enhance the efficacy of 5FU by efficiently targeting the 5FU to in vivo NPC tumors.
Patents obtained
U.S. Provisional Patent Application No 63/267,737 filed on 9 February 2022
Title: Aptamers specific to EBV-associated malignancies and their applications in cancer imaging and therapies
Secured fundings
-
Health and Medical Research Fund (HMRF). Development of molecular probes for surface markers on LMP1-positive nasopharyngeal cancer cells using aptamers selected by cell-SELEX. $ 1,119,496. 2018 -2021.
-
General Research Fund (GRF). Development of Aptamer-Drug Conjugates (ApDCs) for treatment of Epstein-Barr virus (EBV)-associated nasopharyngeal carcinoma. $ 1,172,904. 2022 – 2025
References
-
Rhodes D, Lipps HJ. G-quadruplexes and their regulatory roles in biology. Nucleic Acids Res. 2015;43(18):8627-37.
-
Bishop JS, Guy-Caffey JK, Ojwang JO, Smith SR, Hogan ME, Cossum PA, Rando RF, Chaudhary N. Intramolecular G-quartet motifs confer nuclease resistance to a potent anti-HIV oligonucleotide. J Biol Chem. 1996;271(10):5698-703.
-
Xuan W, Peng Y, Deng Z, Peng T, Kuai H, Li Y, He J, Jin C, Liu Y, Wang R, Tan W. A basic insight into aptamer-drug conjugates (ApDCs). Biomaterials. 2018;182:216-26.